Help
Ethics approval is required for research projects involving human participants, and research activities cannot commence until ethics approval has been provided in writing. Human Research is conducted with or about people, their data, and/or their tissue. In Australia the National Statement on Ethical Conduct in Human Research 2023 (National Statement), is in place to promote ethical human research.
Templates can be accessed at the dropdown menu at the top of this page Help>Templates.
Establish a Profile within EthicsRM
To create a profile within EthicsRM, select the green 'new user' button on the login page.
For all TUA researchers (staff and HDR candidates) the profile is to be based on a TUA email address.
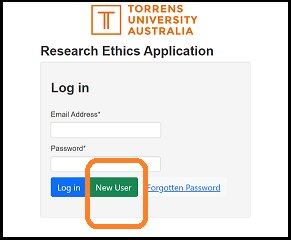
The login page is linked here, or can be accessed from this page at the top black banner
Create and Access a Project
Once logged in using your established profile, you will be taken to the 'Work Area'.
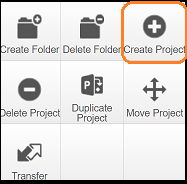
To create a project select the 'create project' icon:
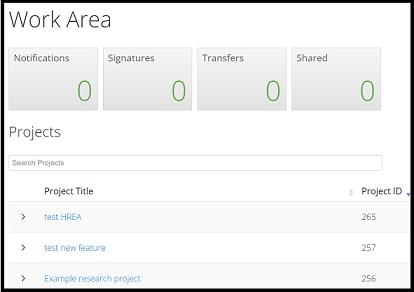
Within the Work Area, all research projects created will be listed by the Project Title and the unique Project ID (ethics application number). To access a previously created project, select the project title hyperlink in blue:
Project Team
At the 'Project Team' section, all research project team members are to be listed.
The position of 'Principal Investigator' has overall responsibility for the ethical management of the research project, and must be a TUA researcher. The position can be allocated by selecting 'Principal Investigator' from the dropdown box at Q1.9.10.
HDR research projects allocate the following positions at Q1.9.10:
- the Principal Supervisor's position is 'Principal Investigator', and
- the HDR Candidate is 'Investigator/Researcher'.
In summary the naming conventions and definitions at Q1.9.10:
- ASSOCIATE/ASSISTANT/SUB/CO-INVESTIGATOR/RESEARCHER: All researchers, apart from the Principal Investigator and the HDR Candidate.
- CHIEF INVESTIGATOR/RESEARCHER: Not used
- CO-ORDINATING PRINCIPAL INVESTIGATOR/RESEARCHER: Coordinating PI for multi-site projects.
- INVESTIGATOR/RESEARCHER: HDR Candidate
- LEAD INVESTIGATOR/RESEARCHER: Not used
- OTHER: if required for RA roles
- PRINCIPAL INVESTIGATOR: Key researcher responsible for the project, allocated to a TUA staff member.
Share an Application
All research team members must have an established profile within EthicsRM.
To share an ethics application with a team member, select the 'share' icon:
![]()
Enter the EthicsRM profile email address for the team member, and select the appropriate access from the available list:
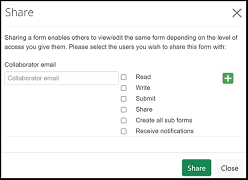
Select the 'Share' green button to complete.
Supporting Documentation
The supporting document to be attached (as part of an ethics application) include, but are not limited to:
At Q4.1
- Project Description
At Q4.2
- (1) TUA Research Endorsement: Coordinated by the Principal Investigator.
- (2) Curriculum Vitae (CV): CV documents for each team member.
- (3) Participant Recruitment: All participant recruitment documentation.
- (4) Participant Information & Consent Form (PICF): PICF in the template format.
- (5) Data Collection: All data collection instruments.
- (6) Authorisations and Permissions: if required, site specific or organisational.
Templates and guides can be accessed at the Help>Templates dropdown at the top of this page.
Declaration Page
The declaration page is delegated to the Principal Investigator (for HDR research projects, this position is held by the Principal Supervisor).

Reviewer Comments
When an ethics application has been reverted, the comments can be accessed by selecting the 'Reviewer Comments' icon:
![]()
Data Collection: Personal Information
At Section 3 of the ethics application, researchers are asked to indicate the type of data they are collecting and using for the research project, and whether the information is categorised as personal, sensitive or health.
The concept of ‘personal information’ is broad under the Australian Privacy Act 1988. The guidance and examples provided by the Office of the Australian Information Commissioner may be helpful in understanding personal information and the types of personal information (such as sensitive information).


